Despite enormous gains in understanding of the mechanisms of disease, we are at a near impasse in new drug development. Most good drug ideas fail at the stage between successful experiments in test tubes and cell culture plates and the attempt to translate the results to intact experimental animals. The failure rate at this stage is so high that some call it the “Valley of Death.” This collapse after much expenditure of time and treasure is hard enough to accept, but loss of a drug in the clinical-trial stage is depressingly common as well, and our clinical-trial system is so encrusted with regulatory impedimenta that enormous amounts of time and money are required to learn that a drug is either a clinical failure or produces only a very small advance. It is very easy to spend a billion dollars to find out that there is very little to show for the effort. In a market-based system such as ours, such losses are nearly impossible to accept because we depend on pharmaceutical company profits and the incredible generosity of donors and taxpayers (who support the National Institutes of Health) to fund the discoveries.
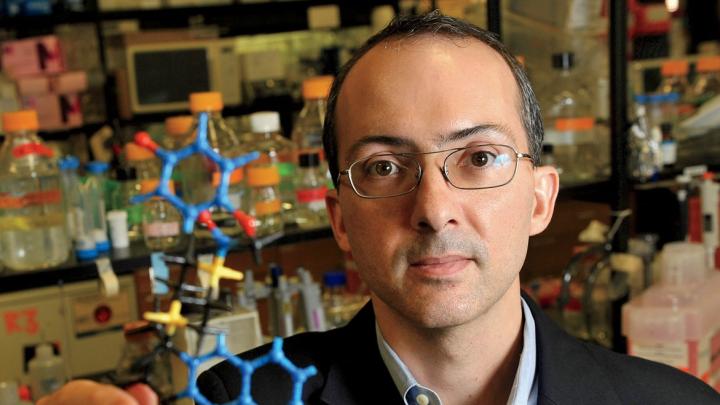
Brent R. Stockwell, Ph.D. ’99, is an associate professor of biological sciences and chemistry at Columbia and a Howard Hughes Medical Institute Early Career Scientist. His scientific pedigree places him in the top echelon of promising chemists, particularly those with a bent to break the worldwide logjam in drug development, the hoped-for end product of the science of pharmacology. In addition to his practical knowledge of and experience with organic chemistry and drug design, Stockwell is a teacher who has produced a very useful small volume, The Quest for the Cure: The Science and Stories Behind the Next Generation of Medicine.
Stockwell begins with a clear statement of the scientific and fiscal challenge that faces patients with severe acute and chronic diseases, the physicians and nurses who care for them, the grantors who support biomedical research, and the pharmaceutical industry. His early chapters explain that much of the reason for the narrowing of the pipeline of drug discovery lies in a vexing dilemma. The cells of a patient or experimental animal contain thousands of proteins, and the cells of each tissue differ from one another based on the specific proteins that they express. Because proteins control cell behavior, the successful treatment of diseases mandates that we produce drugs that will influence proteins. This creates the problem that animates Stockwell: less than 20 percent of the proteins in cells are considered “druggable” (meaning a small chemical or drug can be produced that will bind to the protein and affect its function). The more than 80 percent of cellular proteins that are currently not druggable—because they lack crevices on their surfaces into which a small chemical can bind—remain beyond the reach of today’s pharmaceutical science, even though some of them are prime suspects in the causation of devastating diseases. Stockwell’s goal is to show the reader how such proteins can be made druggable by defining “hot spots” on them (see below), and thereby to advance the field of pharmacology.
Proteins are the engines of the cell. Through their function they control the creation of energy, movement, brain function, death, and reproduction, and they accomplish those functions by making specific contact with one another—in effect, nudging one another into action. It is through the proteins they form that genes make the individual cells in our tissues develop into functioning organs and control the inherited fraction of who we are as individuals. Each protein is a polymer—a long chain of constituent amino acids. The latter are members of a class of more than a score of different small molecules made up of carbon, oxygen, hydrogen, nitrogen, and occasionally sulfur atoms. The order of their insertion into the chains or polymeric structures of individual proteins is directed by one of the approximately 20,000 genes that are composed of DNA and reside on chromosomes in the nucleus of every human cell. Importantly, the precise order of the amino acids in a protein dictates its shape, because the amino acids fold against each other in a three-dimensional pattern that is entirely dependent on their sequence; and the shape of a protein, in turn, dictates its function.
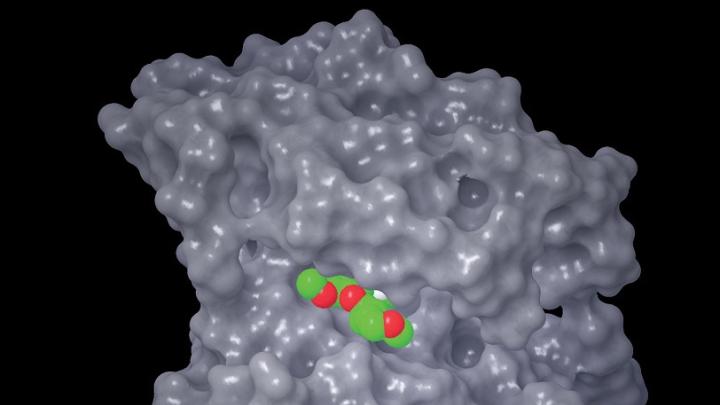
Drugs work by binding to proteins, altering their shape and thereby changing their function. For example, aspirin, a small, readily absorbable molecule and one of the oldest drugs, binds to small crevices on several proteins (including one on blood platelets, thereby rendering them less sticky; thus aspirin taken daily reduces the incidence of heart attacks and strokes). But many proteins, including disease-inducing ones, affect changes in cell function by rubbing up against each other, adhering to a partner protein across large surface areas, thereby altering the shape, and hence function, of each protein.
Small molecules like aspirin may be lost in that huge range of contact. But Stockwell teaches that within the large contact regions there may be “hot spots” where there are tiny but critically important crevices in which a differently configured small molecule might lodge, thus changing the shape of its host and interrupting its abnormal function. Much of the second half of his book reviews the history of and the new approaches to finding those minute crevices and the small molecules that might bind tightly in them. He describes how academic and pharmaceutical-company laboratories prepare vast “libraries” of small molecules and robotically determine which of them may bind to particular proteins that are implicated in disease.
Though Stockwell discusses computer-aided drug design and drug combinations (including his frustrating experience of starting a company to explore the latter) in some detail, he does not address the mechanisms of drug resistance in either cancer or infectious diseases. These are serious omissions because resistance of both infectious agents and cancer cells is dependent on their high mutation rate and the selection for survival of the cells that achieve resistance to one or more drugs. This demands combination therapy. We need to define increasing numbers of useful antimicrobials and anticancer agents just to keep pace as the infectious or malignant cells achieve resistance.
Stockwell refers to “personalized medicine”—the selection of an appropriate drug based on patient-specific gene expression in cancer cells—as he describes the multiple genetic mutations in cancer, but it increasingly seems that the infectious agents and cancer cells are themselves becoming personalized. Cancer, for example, arises from mutation in a single normal cell, but one of its characteristics is instability of DNA and a very high mutation rate. Thus the daughter cells of the originating cancer cell all differ from one another at the genetic level. Accordingly, they are prone to continued mutation and very likely to develop further mutations that render some of them resistant to a particular drug. Dangerous infectious microbes have the same propensity. The populations of microbes or cancer cells must be attacked with multiple drugs to eradicate the invading horde. Hence we will need many drugs to combat them.
Stockwell knows that his readership is unlikely to include many professional organic chemists, so he has adopted a storytelling approach to get his points across. Wisely,his medical case reports are few and far between, because he does not know the details of the patients he describes, but his grasp of the fascinating history of organic chemistry and of the particular experiments that led, for example, to huge gains in our understanding of the protein-driven mechanisms of cancer is firm and more often than not lucidly presented. One may quibble, of course, with some of his tales. His description of the major contributions of Sidney Farber to the birth of cancer chemotherapy, for example, is not quite right. Farber and his colleagues, the first to use a targeted drug (aminopterin) to attack childhood leukemia, were in fact searching for a vitamin to manage the disease, but found that aminopterin is actually an anti-vitamin that kills leukemic cells but is broadly toxic, making precise dosage critical. Selective Toxicity, Adrian Albert’s brief 1950s monograph about antibiotics, well describes that conundrum.
As is true of many good scientists beginning to write for a general readership, Stockwell needs to work on his writing style. His explanations vary from insultingly simple to overly complex. He needs to learn the descriptive methods of Berton Roueché or Atul Gawande. It is important that he do so because his message is very important. He concludes with a call for educating our citizenry on the need for more drugs and for health-research policies that encourage the development of those drugs. In today’s climate of deficit reduction and political stalemate, this seems to be a mournful cry. But Stockwell is absolutely right. Drug development is essential if we are to combat severe diseases, and the hitherto undruggable proteins are the targets we must attack. Despite our current political paralysis, government leaders should listen to Stockwell and be certain to advance our capacity to generate the drugs that our society and the world need.